Atp Is an Energy Carrier. Where Is the Energy Actually Located
How can the hydrolysis of ATP release energy?
We have already seen that chemical energy is released when chemical bonds form .
In biology, we learn that ATP is the energy currency of living things so that it is used to drive those processes on the metabolism that need an energy input. Energy is transferred to metabolic pathways when ATP breaks down into 2 pieces: ADP and orthophosphate.

ATP : the 3 phosphates are on the right
So, are we being told that in biology that the breaking of a bond releases energy? Is this the opposite of what we learn in chemistry? That is a very good question to ask your teachers. Anyway, I will answer it here.
Larger image of the structure of the ATP molecule
The answer:
It is not the actual breakdown of ATP that releases energy. Actually it costs some energy to break that bond, but the processes that follow are the ones which release the energy (in addition to paying back the cost, to initiate the process, of breaking one bond).
The breakage of ATP is a hydrolysis reaction, e.g., it is promoted by water (hydro-water; lysis- break or separate). When ATP is formed we have the opposite process, so that the reaction is a condensation and a water molecule is eliminated.

colour coding for
molecular structures
Following hydrolysis, the phosphate group that is liberated reacts with water and forms orthophosphate (receives the -OH group). The remaining H from the water molecule takes the place of the liberated phosphate, so that ADP is formed.
That means that 2 new bonds have been formed so that some energy is released at these steps. But that is not the whole history. There are also other factors:
1) Getting rid of one phosphate group is energetically favourable because there is a large electrostatic repulsion between phosphate groups in ATP (see picture above) because they are very close together, and all have various negative charges on the oxygens (like charges repel, opposite charges attract).
2) The entropy of the system is increased. Entropy is a measure of the disorganization of systems, and processes that increase entropy tend to be spontaneous. Chemical reactions whose products are more disorganized than the reagents are favoured (but this is not the only condition, there are also enthalpy considerations- both factors are taken into account in the Gibb's free energy). The hydrolysis of ATP increases disorganization because you start with one piece and end up with two.
3) Chemical reactions are also favourable when they produce highly stable products. The orthophosphate group produced from the hydrolysis of ATP is highly stable because it presents various resonance structures. That means that the double bond can move to different positions in the molecule, causing rearrangements of charge. Stability is gained in this process. This is explained by quantum mechanics.
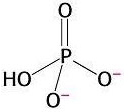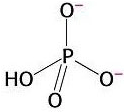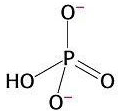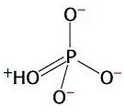
Resonance in
orthophosphate
look at resonance in other molecules (external link)
So this are some of the answers to the question. It is a tricky question. I hope I could convince you!
Atp Is an Energy Carrier. Where Is the Energy Actually Located
Source: https://sci-culture.com/advancedpoll/GCSE/atp.php
0 Response to "Atp Is an Energy Carrier. Where Is the Energy Actually Located"
Post a Comment